CLINICAL TESTING
Our hygiene and protection products are tested under dermatological control for sensitive skin and under paediatric and ophthalmological control to ensure non-aggressive formulas, conceived with cutting-edge technology and developing a perfect texture.
With the Osmè Baby & Kids products, say goodbye to tears and stinging eyes, to dry skin and redness, with our formulas clinically tested under dermatological control for sensitive skin, and under paediatric and ophthalmological control. The cleansing products and creams have also undergone efficiency tests to assess their moisture content.



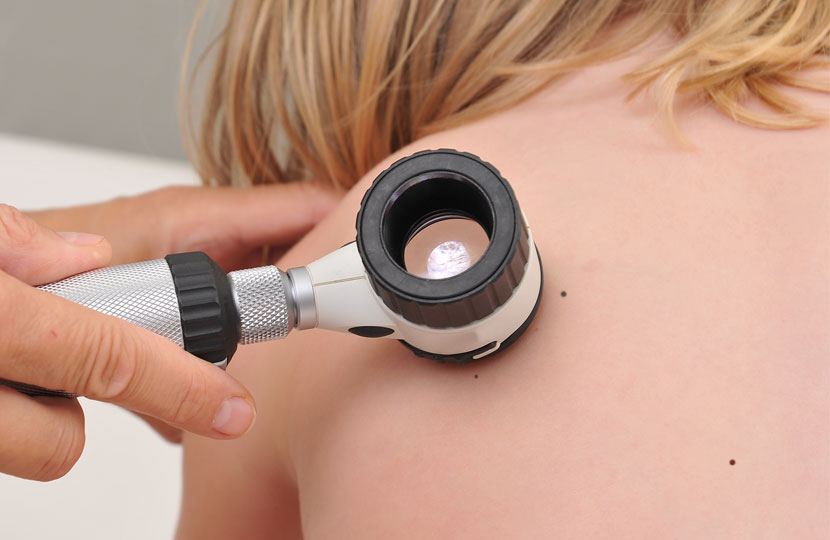
PAEDIATRICIAN TEST: EVALUATING YOUR BABY SKIN TOLERABILITY
AIM OF THE STUDY: Product tolerability is tested through the analysis of side effects that may occur during the product use (erythema, desquamation, dryness)TEST EXECUTION: The parents visit the ambulatory with their babies after 15 and 30 day of application. At each follow-up visit a dermatological examination was done for evaluating the occurrence of skin discomforts.
PRODUCT APPLICATION METHOD: The product has been applied every day for 4 weeks following the instructions given for each product category.
MONITORED CHECKS AND DERMATOLOGICAL EVALUATIONS: Assessments at baseline, after 15 days and after 30 days of the characteristic skin reactions to baby skin care products: Erythema, Dryness and Desquamation (tactile roughness, flakiness); experimenter takes care to notice the presence of others clinical features of the skin. The skin assessments listed are made using a rating scale with the following designations: 1= none; 2= mild; 3= moderate; and 4= severe.
This test was conducted for all the osme baby and kids skincare products with a result of "high tolerability, clinically tested under paediatric control"

Clinical tests panel of 20 babies with healthy skin under conditions of normal use aged between 1 month and 5 years for 30 days.
NO TEARS TEST: EVALUATING THE OCULAR TOLERABILITY
AIM OF THE STUDY: The test allows to assess whether the tested cosmetic product is tolerated in the eye area in comparison with a reference product claimed as “does not sting the eyes”.TEST EXECUTION: This is a comparative analysis between the tested product and the reference product. The protocol foresees the instillation in one eye of the product under study, compared with a reference product already tested and considered as not stinging the eyes, applied in the same conditions in the other eye to the same subject.
OPHTHALMOLOGICAL EVALUATIONS: Before and after product instillation ophthalmologist evaluates the status of the eyes.
STINGING EFFECT EVALUATION: Duration and intensity (bigger than 0, no stinging sensation) of the stinging felt by the volunteers are scored according to different values from very weak (1) to very strong (8).
TEARING EFFECT: The tearing score is evaluated as following “Score after instillation minus Score before instillation”.
For the osmè baby & kids cleanser the percentage of subject showing a tearing effect bigger than 0 is lower than 15% that the tested product can claim on the label “no tears”.

Clinical tests panel of 15 volunteers (agedover 18) with sensitive eyes for maximum 3 minutes
EFFICACY TEST: EVALUATING THE IMPROVING OF SKIN HYDRATION
AIM OF THE STUDY: The test aim is the evaluation of the improving of the skin after the application of a cosmetics product using instrumental techniques as stratum corneum hydration measurement.TEST EXECUTION: The area have been defined with a marker, for each volunteers where the product will be applied. For the measurement of the hydration level of the horny layer we have used the instrument Corneometer.
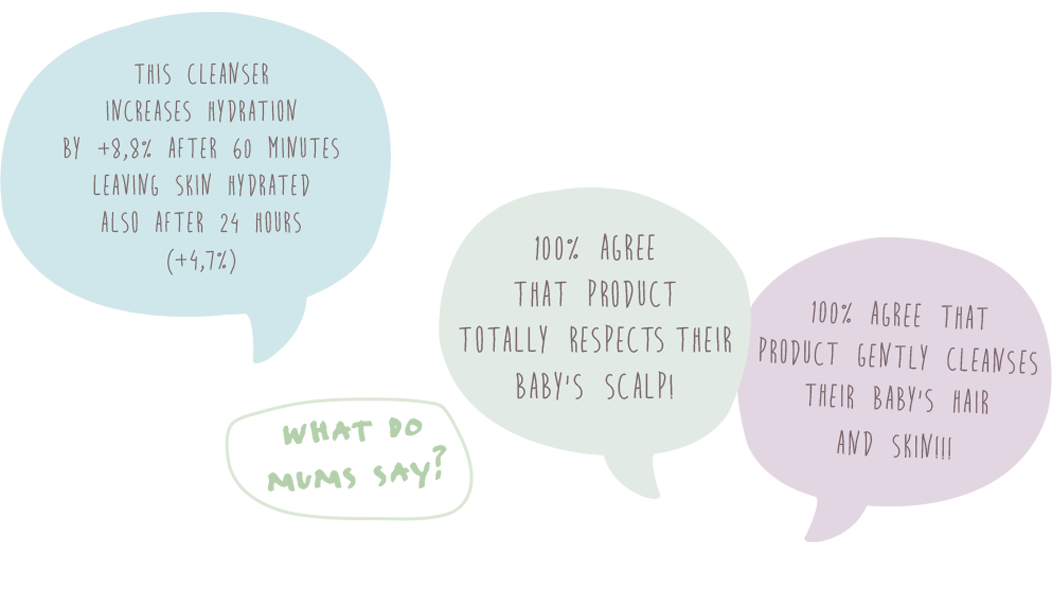
This test was conducted with a positive result for both bath and care products. In specific here the results: for the clenser we can say that increases hydration by +8,8% after 60 minutes leaving skin hydrated also after 24 hours (+4,7%) and for the cream we can say that increases hydration by +35,5% after 60 minutes leaving skin hydrated also after 24 hours (+11,3%).

Clinical tests panel of 20 adults for 24 hours.

Loading